RAD140 recipe: how about homemade rad140 solution with Ethanol?
When it comes to experimenting with selective androgen receptor modulators (SARMs) like RAD140, understanding solubility is critical. Solubility not only influences the preparation process but also plays a role in absorption and bioavailability when taken. Today, we’re diving into the solubility test of 1g of RAD140 powder in ethanol, detailing every step and observation along the way.

Why Test Ethanol as a Solvent?
Ethanol is a commonly used solvent in research and pharmaceutical settings due to its versatility and safety. It's known to dissolve many organic compounds effectively, making it a popular choice for SARMs like RAD140. Additionally, its ability to evaporate quickly makes it ideal for applications where solvent removal is necessary. Before diving into the process, we hypothesized that ethanol would likely demonstrate decent solubility for RAD140, though not necessarily as efficient as solvents like DMSO.
Step-by-Step Testing Process
Initial Addition (1ml):
We started with 1ml of ethanol, adding it to the beaker containing 1g of RAD140 powder. After shake it to help the solvent mix, we found 1ml of ethanol is too little, there’s no clear sign of dissolution, though the RAD140 has become damp.

2ml
We’ll add another 1ml , making it 2ml in total. After shaking to enhance mixing, we still don’t see completely dissolution.
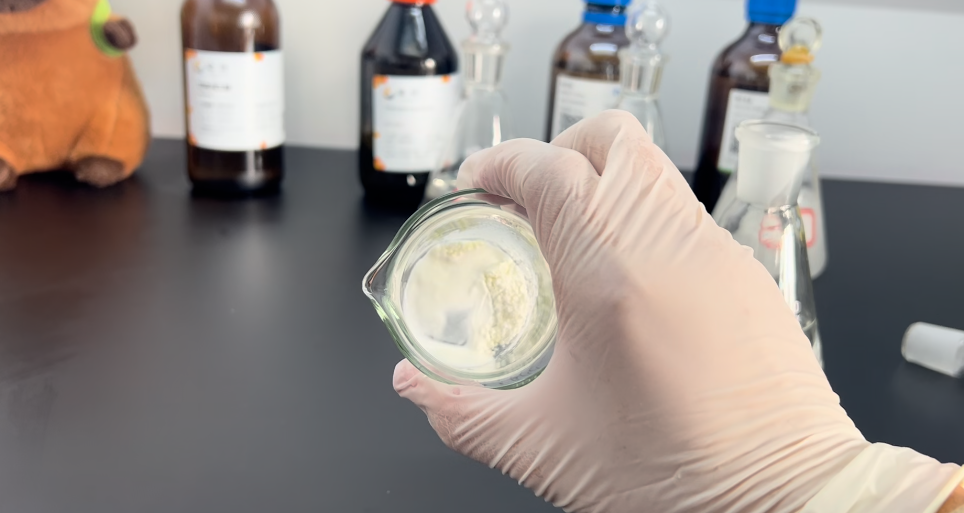
3ml
Adding 1ml more, brings us to a total of 3ml.
At this point, the RAD140 powder has mostly turned into a liquid-like state.

4ml
Now, let’s increase the solvent ethanol to 4ml and see what happens.
--RAD140 still hasn’t fully dissolved, but the liquid is more fluid. It’s a much better state compare to the water solvent test.

5ml
From what we can see so far, only a small portion of the powder has dissolved. It’s likely because there isn’t enough solvent yet.
We’ll add in another 1ml ethanol, bringing the total to 5ml.
Usually, after dissolution, the liquid is in a relatively clear and transparent state.
After stirring, the liquid has turned into a cloudy milky-white state, but there’s no obvious dissolution. It seems we’ll need to keep adding ethanol to further explore how well it dissolves RAD140.

6ml
Let’s add another 1ml of ethanol, so there is 6ml in total now.
As usual, we stir the mixture to help fully combine. Still, no significant dissolution is observed.

7ml
Next step is 7ml ethanol. After stirring, we’re still seeing a mixed state of the sample and solvent.

8ml
Adding another 1ml, bring us to 8ml of ethanol. After stirring, we can clearly notice that the mixture is more flow than before.
9ml
Alright, let’s add another 1ml, and 9 ml of ethanol. The fluidity improve further.
10ml
Adding one more ml let’s take a closer look at the liquid. Now we are at 10ml of ethanol, and her is the state:
The liquid appears milky white with good flow, but it seems that only a small portion of the RAD140 powder has dissolved.

Next, we will increase the ethanol to 20ml to see if there is any significant changes in the solution.
Increased Ethanol (20ml):
An 10ml of ethanol was added, bringing the total to 20ml. After stirring for another 5 minutes, and now we’ll check the solution status of the liquid.
As you can see, increasing the ethanol hasn’t made much of a difference. The liquid remains cloudy and milky in appearance

Further Addition (50ml):
For the final test, we’ll add ethanol up to 50ml and stir. Since ethanol hasn’t show great solubility so far, we’ll let the mixture sit for 3 minutes to see if the RAD140 powder settles or precipitates.

After 3 minutes, we can clearly see the liquid has separated into layers, with most of the RAD140 powder settling at the bottom of the container. There’s still a small amount of RAD140 suspended in the solution. But overall, its solubility in ethanol is slightly better than in water.

Conclusion
The solubility of 1g of RAD140 in ethanol was tested systematically, until adding 50ml solvent ethanol, 1g powder still didn’t dissolving totally. That means ethanol Partially dissolve RAD140.
Ethanol is not a good solvent compared to DMSO and PEG300, but ethanol is a relatively easy solvent to obtain. If you do not require the concentration of RAD140 solution, it may be suitable. However, it should be noted that ethanol is volatile, and it is necessary to refer to this point when storing the ethanol solution of RAD140.
